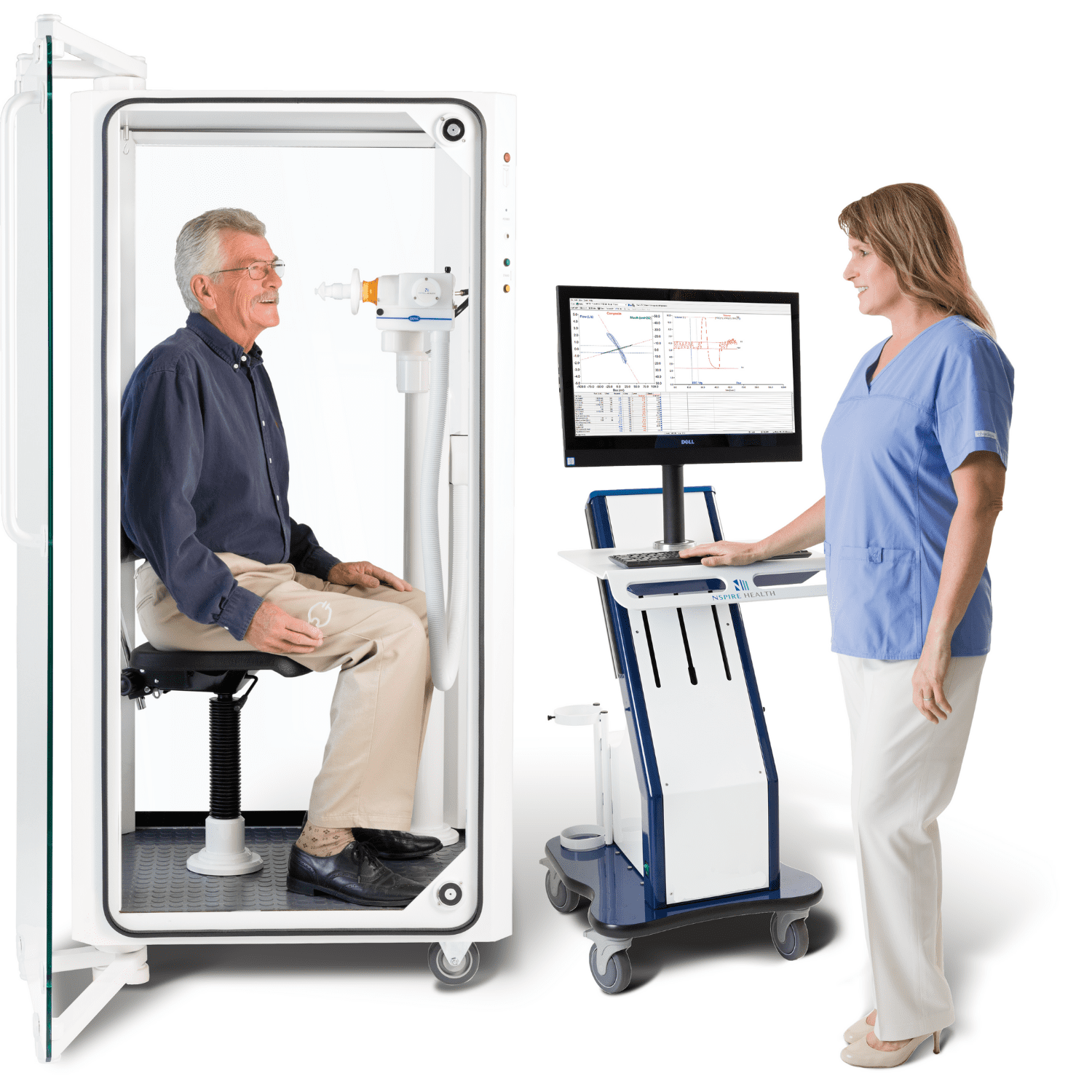
New Product Launches and Strategic Partnerships To Boost Pulmonary Function Testing Devices Demand Globally
Growing need for screening non-COVID patients and the rise in the occurrence of chronic respiratory ailments such as pulmonary emphysema, asthma, pulmonary fibrosis, and chronic obstructive pulmonary disease have boosted demand for pulmonary function testing devices. With a growing demand for pulmonary function testing to assess respiratory ailments, the market for pulmonary function testing devices is predicted to gain traction in the years to come. Escalating preference for minimally invasive testing procedures has enhanced the application of pulmonary function testing devices in the healthcare industry. According to the report published by Allied Market Research, the global pulmonary function testing devices market is anticipated to garner massive returns in the years ahead.
Key participants in the global pulmonary function testing devices market are executing strategies such as the launching of new products and technologies with a view of contributing sizably towards patient care and improving the quality of life of the patients. In addition, the new product and technology launches will help the industry players in expanding their customer base and surge their profits. Apart from this, strategic partnerships are likely to help industry players in expanding their product lines along with product portfolio expansion and increasing product width. Thus, the execution of this key business strategy will assist the industry players in expanding their business reach across the globe and fulfill consumer demand for innovative pulmonary function testing devices. This, in turn, will create new horizons of growth for the pulmonary function testing devices market globally. Firms in the pulmonary function testing devices business are acquiring other firms in the same industry or other industries for strengthening their business portfolio along with establishing a strong foothold in the global market. These strategies are projected to help market players in generating new revenue streams for their business and explore new markets with huge untapped growth potential. In addition, the strategy will also assist industry participants in diversifying their business requirements, thereby increasing their profits. Let us discuss some of the major trends witnessed across the globe and leverage the growth of the global pulmonary function testing devices market.
New product and technology launches have favorably impacted the growth of every industry and the global pulmonary function testing devices market is one of them. Vitalograph, a global leader in the respiratory diagnostics industry, launched a new generation pneumotrac spirometer and upgraded spirotrac 6 software in the U.S. Reportedly, in integration with spirotrac PC software, the new generation pneumotrac spirometer becomes a powerful tool for diagnosing respiration in children and adults. Moreover, users can gather reliable results from the respiratory tests carried out by the device. In addition, pneumotrac spirometer can also connect easily with an electronic medical records system for streamlining the clinical workflow process. The strategic move of the new medical device launch is aimed at improving respiratory diagnosis across the U.S. Moreover, Respira Labs, a U.S.–based respiratory healthcare tech startup firm, introduced an artificial intelligence-driven wearable lung monitor named Sylvee. The new pulmonary function testing device makes use of acoustic resonance for monitoring lung function and determining changes in lung air volume. The move is aimed at diagnosing COVID-19, chronic obstructive pulmonary disease, and asthma in its initial stage.
Continuing with this trend of new product and technology launches, Cipla Limited, an India-based pharmaceutical firm, introduced the Spirofy device in India for testing lung function. For the record, Spirofy is wireless, portable equipment that can perform lung function tests in remote areas and outdoors in India. The move is aimed at diagnosing people with asthma and chronic obstructive pulmonary disease along with other lung ailments. The initiative will also help in improving the lives of patients through cost-effective and accurate diagnosis of respiratory disorders in India. Furthermore, ndd Medical Technologies, Inc., a key provider of easy-to-use pulmonary function testing equipment, launched a single patient-use inline filter for its pulmonary function testing equipment for detecting COVID-19 virus in patients. The strategic move will help healthcare service providers in effectively diagnosing and treating lung disease with precision. Moreover, the filters will complement ndd’s range of pulmonary function testing devices such as EasyOne Air, Pro LAB, and Pro. Furthermore, Vyaire Medical, Inc., a key medical technology manufacturer based in the U.S., declared that it received approval from the U.S. Food and Drug Administration (FDA) for its pulmonary function testing technologies including Vyntus BODY and Vyntus ONE running on the SentrySuite Software tool. The move is aimed at bringing innovation in the respiratory diagnostics domain within the next few years.
Strategic partnerships have played a pivotal role in the expansion of the medical device industry and the global pulmonary function testing devices industry is no exception to this. VitalFlo, a predictive and preventative respiratory health firm, and Vitalograph joined hands for offering smart respiratory health monitoring solutions through remote monitoring spirometry devices of Vitalograph. The initiative will help patients in performing lung function tests effectively from anywhere and anytime, thereby helping patients effectively manage their respiratory health. Moreover, the move is intended at offering innovative respiratory health solutions to the people and enhance the quality of life of the patients.
With the ongoing trend of entering strategic partnerships, KoKo, LLC, a respiratory information systems software and medical device manufacturer, and EirMed, a key medical equipment producer, joined hands. The partnership is intended for streamlining the production of respiratory medical equipment such as pulmonary function testing devices for effective patient care. CB Scientific, Inc., a key producer of non-invasive ambulatory cardiac monitoring devices, and Shenzhen Pump Medical Co., Ltd., a key medical device manufacturer, joined hands. As per the terms of the cooperative manufacturing agreement between the two firms, Shenzhen Pump will be recognized as a qualified producer of record for CB Scientific’s products such as the myCam cardiac event monitor, We Chat smartphone app, and interactive cloud-based acquisition software in China. The move will help CB Scientific in expanding its medical device portfolio in China. The initiative will also contribute notably toward the growth of pulmonary function testing devices market size in the country.
Firms in pulmonary function testing devices manufacturing business are trying to expand their product portfolio by successfully executing mergers & acquisitions strategy. SD Biosensor and SJL Partners signed a merger agreement for acquiring Meridian Bioscience, the manufacturer of a diagnostic product, for nearly $1.53 billion in an all-cash transaction. Reportedly, Meridian Bioscience manufactures, promotes, and distributes a slew of medical diagnostic devices for domains such as blood lead level testing, upper respiratory infections testing, and gastrointestinal testing. The move will help in reinforcing the product portfolio of SD Biosensor and Meridian Bioscience. It will also contribute notably toward the expansion of the global pulmonary function testing devices market.
Continuing with the strategy of mergers & acquisitions, Novartis’s Sandoz acquired Coalesce Product Development, a UK-based medical equipment and drug development firm. The strategic move is aimed at expanding and strengthening the respiratory drug and complex generics portfolio of Sandoz. Furthermore, MGC Diagnostics Corporation, a medical technology firm in the cardiorespiratory health solutions business, and Altus Capital Partners, Inc., a private equity firm, acquired Lemon Medical, GmbH, a medical technology firm. The initiative is predicted to help MGC Diagnostics Corporation further strengthen its in-house production capabilities and contribute notably to its product offerings. Industry players are implementing the strategies of new product & technology launches, partnerships, and acquisitions for increasing their consumer base across the globe. Apart from this, these strategies will help these players in contributing substantially toward market revenue along with enhancing return on investments.
We are always looking for a new writer for guest blogging. In order to give you high quality exposure to the readers all around the world.